Understanding the formal charge of atoms within a molecule is a foundational concept in chemistry that lets us predict the stability of molecules and the way they interact with each other. It’s a way of keeping track of the electrons, particularly in complex molecules where the sharing of electrons can be uneven. Whether you are a student, educator, or just someone with a keen interest in chemistry, grasping this concept is valuable. Let’s embark on a chemistry adventure to decode the formal charge and its calculation with straightforward guidance!
The Basics of Formal Charge
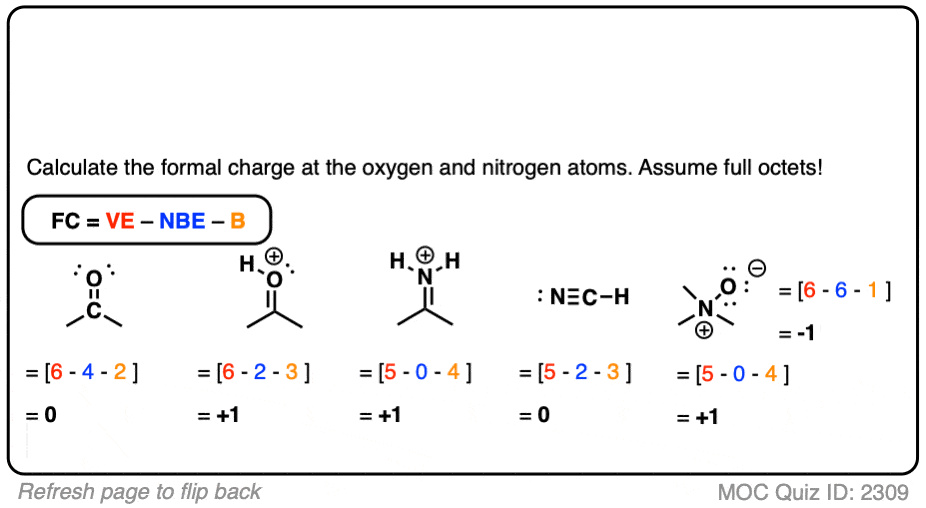
Accurately assigning a formal charge to an atom is a critical step in understanding the behavior of molecules in chemical reactions. The formal charge is the theoretical charge on an individual atom within a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electronegativity. This concept helps chemists determine the most likely structure of a molecule.
Detailed Steps
- Identify the Valence Electrons: Determine the number of valence electrons for the atom in its elemental state, often found in the periodic table.
- Count the Nonbonding Electrons: Find the total number of nonbonding (lone pair) electrons on the atom in the molecule.
- Count Bonding Electrons: Count the electrons the atom shares with others in bonds. Each bond to the atom contributes two electrons.
- Calculate the Formal Charge: The formal charge is found by taking the number of valence electrons in the free atom, subtracting the number of nonbonding electrons, and subtracting half the number of bonding electrons.
Summary
This method is crucial for visualizing how electrons are distributed in a molecule, which affects reactivity. Its limitation is that it doesn’t account for the differences in electronegativity between atoms.
Electron Bookkeeping
To understand electron distribution, chemists use a “bookkeeping” method to keep track of electrons in molecules. This method is like balancing a checkbook but for electrons.
Detailed Steps
- Total Valence Electrons: Start with the total valence electrons of the free atom.
- Assign Electrons in Covalent Bonds: Assign one electron to the atom for each covalent bond it forms.
- Allocate Lone Pair Electrons: Allot all the lone pair electrons to the atom.
- Perform the Calculation: The formal charge equals the valence electrons of the atom minus the sum of lone pair electrons and the one electron per bond.
Summary
The bookkeeping method helps differentiate between different possible structures. However, it’s a simplification and may not always perfectly represent a molecule’s true electron distribution.
Lewis Structures
Creating Lewis structures is a visual way to see the bonding between atoms and the lone pairs of electrons in a molecule.
Detailed Steps
- Draw the Lewis Structure: Sketch the molecule showing bonds between atoms and lone pairs.
- Count Bonds and Lone Pairs: Identify the number of bonds to each atom and the number of lone pairs.
- Use the Formal Charge Formula: Apply the formula using the information from the Lewis structure.
Summary
It’s especially beneficial for visual learners and simplifies comparisons between different structures. The downside is that drawing complex molecules can be challenging and error-prone.
Resonance and Formal Charge
Resonance structures show molecules where the distribution of electrons can change without altering the arrangement of the atoms, which affects the formal charge of atoms within these structures.
Detailed Steps
- Draw Resonance Structures: Represent all viable resonance variants of the molecule.
- Assign Formal Charges: Determine the formal charge for each atom in every resonance structure.
- Compare Charges: Evaluate which structures have charges closest to zero, as these are often more stable.
Summary
Understanding resonance structures gives a more realistic picture of electron distribution, but interpreting resonance can be complex.
Using Oxidation States
Although not the same as formal charge, understanding oxidation states can sometimes help in understanding the context of formal charge, especially in ionic compounds.
Detailed Steps
- Determine Oxidation Numbers: Using rules for oxidation states, assign these to each atom.
- Relate to Formal Charge: Compare the oxidation state to the expected valence electron count to infer formal charge.
- Understand the Difference: Recognize that oxidation states reflect electronegativity differences, while formal charge does not.
Summary
Oxidation states offer additional insights but may not always align with the concept of formal charge.
The Charge Distribution Puzzle
Seeing a molecule as a puzzle where you have to distribute charges can aid in understanding the formal charge concept.
Detailed Steps
- Consider All Atoms: Look at the molecule’s total valence electrons and its overall charge.
- Distribute Electrons: Divide the electrons according to bond formation and lone pairs.
- Apply the Formal Charge Formula: Calculate the formal charge for each atom.
Summary
This method turns the learning process into a more engaging activity but may oversimplify some aspects of the distribution.
Trends in the Periodic Table
Understanding how formal charges tend to occur across the periodic table can help predict the formal charge of atoms in a molecule.
Detailed Steps
- Note Periodic Trends: Recognize general trends for valence electrons.
- Apply These to Molecules: Judiciously use these trends when evaluating formal charge.
- Cross-check with Calculation: Confirm findings with a formal charge calculation.
Summary
Periodic trends provide a helpful heuristic, although they’re not a substitute for actual calculations.
Conceptual Understanding Before Calculation
A thorough understanding of what formal charge represents aids in its calculation and understanding molecular stability.
Detailed Steps
- Learn the Concept: Dive into the theory behind formal charge.
- Practice on Simple Molecules: Start with easy calculations for rudimentary molecules.
- Progress to Complex Molecules: Gradually work on applying the concept to more intricate structures.
Summary
Deep conceptual understanding builds a strong foundation, though it requires time and patience.
Using Software Tools
Modern chemistry software can help visualize and calculate the formal charge automatically.
Detailed Steps
- Choose a Chemistry Software: Pick a reputable chemical modeling tool.
- Input the Molecule: Enter the structure into the software.
- Analyze the Results: Let the tool calculate the formal charge and analyze the distribution it suggests.
Summary
This offers a fast and accurate calculation but may deprive learners of the foundational understanding of doing it manually.
Practice Problems
Applying knowledge through practice problems reinforces the concepts and calculations associated with formal charges.
Detailed Steps
- Find Practice Problems: Use textbooks or online resources to find problems.
- Work Through Problems: Calculate formal charges and check answers.
- Review Mistakes: Understand errors in calculations to improve.
Summary
Practical application enhances learning but may initially be frustrating if the concept isn’t fully grasped.
In conclusion, calculating formal charge is like piecing together a puzzle where each atom’s electrons must be accounted for. Whether using traditional pen and paper methods, drawing out Lewis structures, or utilizing modern software, each approach has its place in education and practice. Above all, understanding the implications of formal charges in molecules is crucial for anyone delving into the intricacies of chemistry.
FAQs
-
What is a formal charge?
A formal charge is a theoretical charge assigned to an atom in a molecule, calculated by assuming that electrons in chemical bonds are shared equally between atoms. -
Why is it important to calculate formal charges?
Calculating formal charges is important to predict the stability, reactivity, and the most likely structure of a molecule, which is essential in the study of chemical reactions. -
Are the formal charge and oxidation state the same thing?
No, they are different concepts. The formal charge assumes equal sharing of electrons, regardless of actual differences in electronegativity, whereas the oxidation state takes into account electronegativity differences.







