Understanding the concept of molar equivalents is an essential part of chemistry that deals with the stoichiometry of reactions. It’s a way of expressing how many moles of one substance react with another, providing critical insight into the proportions needed for a chemical reaction. This guide aims to demystify the process of calculating molar equivalents for those who may not have a strong chemistry background.
Understanding Molar Mass
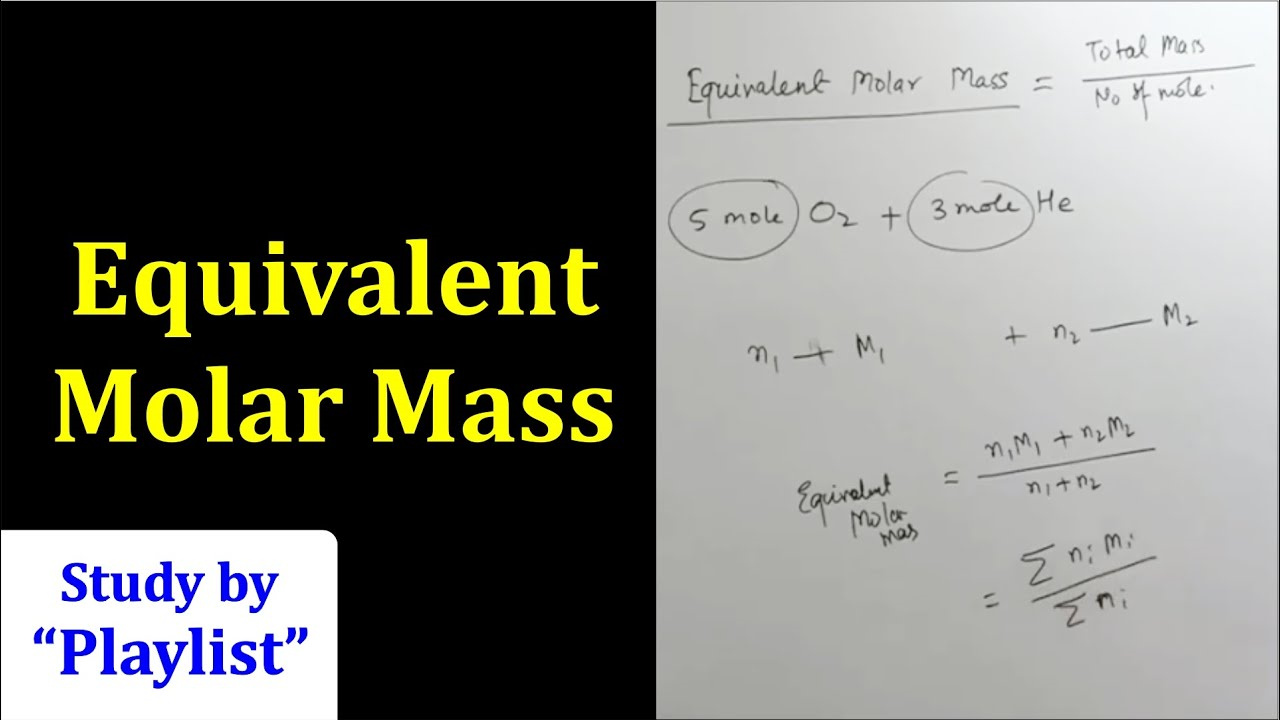
Before diving into molar equivalents, it’s important to understand molar mass. Molar mass is the weight of one mole (6.022 x 10^23 particles) of a substance, usually expressed in grams per mole (g/mol). Knowing the molar mass of a substance is crucial for calculations in chemistry.
Detailed steps:
- Locate the element(s) on the periodic table to find their atomic weights.
- For compounds, add the atomic weights of each element in the formula, considering the number of atoms of each.
- The sum represents the molar mass of the substance in grams per mole.
Summary:
Familiarizing oneself with molar mass is an essential first step. It’s straightforward, requiring only basic arithmetic, but it’s critical for ensuring accuracy in subsequent calculations. The downside is that it can be tedious when dealing with complex molecules.
Moles to Molar Equivalents
Once you understand molar mass, you can start converting moles into molar equivalents.
Detailed steps:
- Determine the molar mass of the substance.
- Understand the concept of equivalence, which relates to the substance’s role in a reaction.
- Calculate the molar equivalent by considering the stoichiometry of the reaction.
Summary:
This conversion is a fundamental skill in chemistry. It allows you to work out the exact amounts of reactants needed for a reaction, reducing waste. However, mistakes here can lead to incorrect proportions and reactions failing to proceed as expected.
Using a Balanced Equation
A balanced chemical equation is pivotal in understanding molar equivalents because it reflects the stoichiometry of the reaction.
Detailed steps:
- Balance the chemical equation.
- Use the coefficients of the reactants and products as a ratio to determine molar equivalents.
- Apply the ratio to the moles of the substance you’re using to find the equivalents.
Summary:
This approach is at the heart of chemistry. It allows for precise calculation of reactants and products, optimizing reaction conditions. However, balancing equations can be challenging for complex reactions.
Ratio Method
The ratio method is used to relate amounts of reactants in a balanced equation.
Detailed steps:
- Write down the balanced equation.
- Identify the ratios of the substances involved.
- Apply these ratios to the quantity of interest.
Summary:
This method simplifies the relationship between reactants and products. Misunderstanding the balanced equation, however, can lead to incorrect calculations.
Using Equivalency Factors
Equivalency factors are numbers that convert moles to equivalents based on the substance’s role in the reaction.
Detailed steps:
- Determine the equivalency factor of the substance.
- Multiply moles by the equivalency factor to find molar equivalents.
Summary:
This can simplify calculations drastically but requires a correct understanding of the reaction’s stoichiometry.
Dimensional Analysis
Dimensional analysis is a versatile method used in various chemistry calculations, including molar equivalents.
Detailed steps:
- Write down what you know, including the molar mass and moles of the substance.
- Set up conversion factors to cancel out unwanted units.
- Perform the calculations to find equivalents.
Summary:
It’s useful for complex conversions and reinforcing understanding of moles and molar mass, with a potential downside of being abstract for new learners.
Limiting Reactant Consideration
In chemical reactions with multiple reactants, it’s essential to identify the limiting reactant to calculate molar equivalents accurately.
Detailed steps:
- Calculate the moles of all reactants.
- Determine the limiting reactant based on stoichiometry.
- Use the limiting reactant’s moles to calculate equivalents.
Summary:
This method ensures efficient use of reactants and identifies reaction restrictions, but it can be complex for multistep reactions.
Online Calculators
Online calculators can quickly determine molar equivalents without extensive manual calculation.
Detailed steps:
- Search for a reputable online molar equivalent calculator.
- Input the required information, like molar mass and stoichiometry.
- Interpret the calculator’s output for molar equivalents.
Summary:
These tools are convenient and user-friendly, but reliance on technology may inhibit a deeper understanding of the underlying concepts.
Concept Reinforcement with Practice Problems
Practice is essential in mastering the calculation of molar equivalents.
Detailed steps:
- Find practice problems from reputable sources.
- Apply the methods described above to solve them.
- Check answers with a solution guide or tutor.
Summary:
Practice enhances skills and confidence but requires time and dedication.
Common Mistakes and Troubleshooting
Awareness of common errors can prevent them in future calculations.
Detailed steps:
- Double-check for arithmetic errors.
- Ensure chemical equations are balanced.
- Verify unit conversions are accurate.
Summary:
Identifying and learning from mistakes is crucial for mastering calculations, though it might be discouraging initially.
In conclusion, calculating molar equivalents doesn’t have to be intimating. By breaking down the process into understandable concepts and methodical steps, even those new to chemistry can grasp these calculations. Remember, practice and attention to detail will enhance your understanding and proficiency.
FAQs
-
What is the difference between moles and molar equivalents?
- Moles measure the quantity of a substance, while molar equivalents relate this amount to its participation in a chemical reaction.
-
How do I find the equivalency factor?
- The equivalency factor is based on the substance’s functionality in the reaction, often found by looking at the balanced equation.
-
Can molar equivalents be used for all types of reactions?
- Yes, they are applicable to all reactions, but the approach may vary depending on the complexity of the reaction.









